Continuous Improvement in Medical Device Manufacturing
At Salt Medical, we understand that excellence in medical device manufacturing doesn’t come from a one-time achievement, it’s built through continuous improvement. In an industry where precision, compliance, and quality are non-negotiable, refining how we design and manufacture is essential to maintaining both innovation and safety.
Why Continuous Improvement Matters
Manufacturing medical devices is inherently complex. Each product may require multiple precision-engineered parts, some small, delicate, and critical to function. Every step in the process must adhere to strict regulatory standards, including sterilization protocols, traceability, and documentation.
Continuous improvement in this context means committing to ongoing enhancements across the board, without compromising patient safety or regulatory compliance. That’s why at Salt Medical, all changes follow our quality management system and are thoroughly validated.
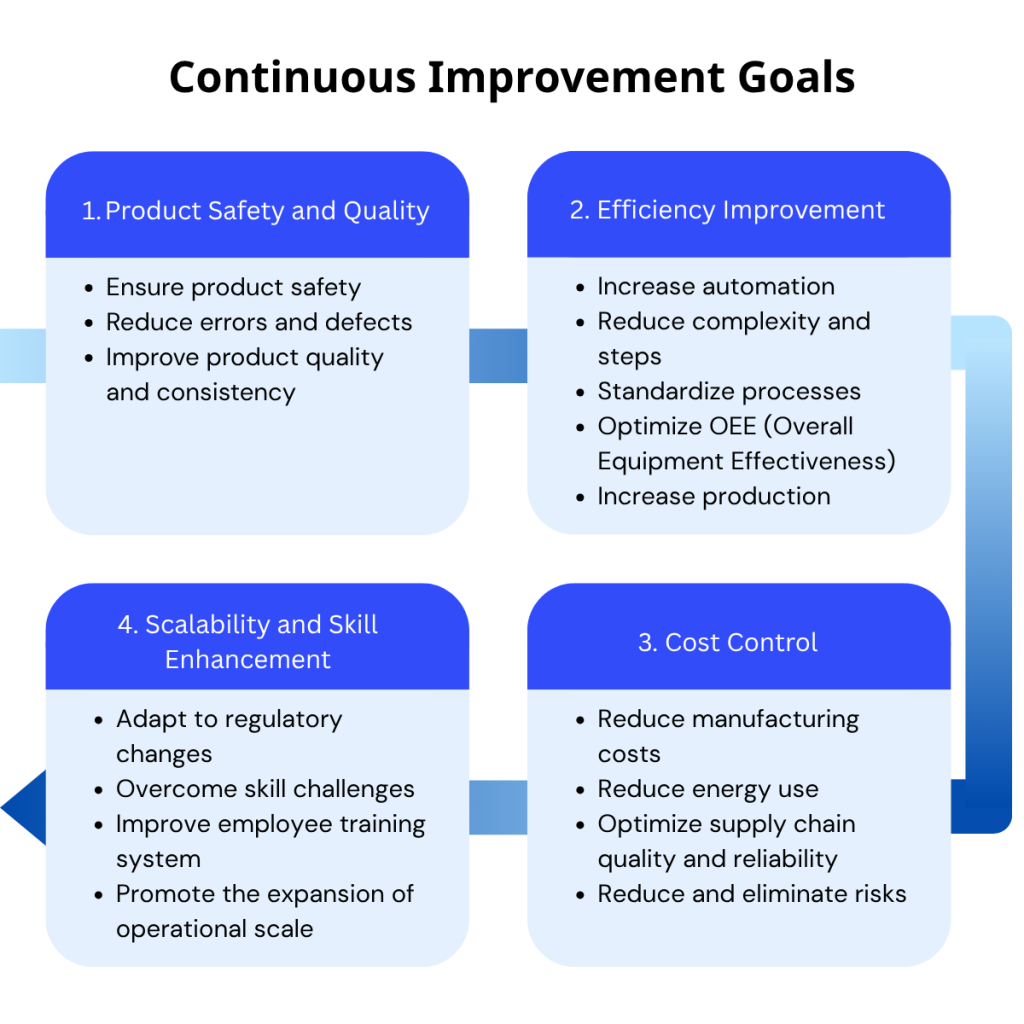
Figure 1: Four pillars of continuous improvement
Our Key Focus Areas
To achieve this, we identify and optimize key “entry points” in the manufacturing process—targeting areas with the greatest potential for impact while maintaining strict quality controls.
These areas include:
- Product quality and consistency
- Packaging and labelling
- Operator training and skills
- Equipment reliability
- Process standardization
- Raw materials and supply chain resilience

Figure 2: Key opportunities for continuous improvement in medical device manufacturing
The Goals Behind Our Strategy
At Salt Medical, our continuous improvement strategy aims to:
- Ensure patient and product safety
- Reduce errors and product defects
- Simplify complex processes and reduce steps
- Adapt quickly to changing regulations
- Improve training and close skill gaps
- Promote scalability and prepare for future growth
- Optimize energy use and overall cost-efficiency
- Improve OEE (Overall Equipment Effectiveness)
- Build a more robust, agile supply chain
By focusing on these areas, we increase production output, enhance product quality, and reduce risk delivering better value to our clients and improving patient outcomes globally.
Check out our last blogs:
- How Computational Modeling and Simulation Are Powering the Next Wave of Medical Device Innovation
- Transforming Vision into Reality: Why CDMOs are Reshaping Medical Device Innovation
- Transforming Vision into Reality: Why CDMOs are Reshaping Medical Device Innovation
- The Road to Success in Medical Device Manufacturing Series 4: Partners
- Why Prototyping Is the Key to MedTech Innovation, and How Salt Medical Helps You Get It Right







