In recent years, the medical device industry has developed rapidly, and the complexity of product development and manufacturing has gradually increased. In this context, CDMO (Contract Design and Manufacturing Organization) provides companies with end-to-end support from concept development to commercial production through its professional capabilities and comprehensive services, becoming an important force in promoting medical device innovation.
So, what value can CDMO bring to medical device companies? How to choose a suitable CDMO? This article will give you detailed answers

The road to success in medical device manufacturing Series 4: Partners (Figure 1)
What is CDMO?
Medical device contract design and manufacturing organizations (CDMOs) provide end-to-end services throughout the medical device product lifecycle, from the development of the initial concept through all stages of the design process to the creation of working prototypes ready for testing and clinical trials. CDMOs often provide regulatory support as well.
In addition, there are obvious differences between CDMO and CMO. CMO is a contract manufacturing organization. Traditional CMOs do not participate in the design and development process, which is critical to many projects and medical device companies. CDMOs, on the other hand, are deeply involved in the design and development phase, providing companies with more comprehensive and flexible solutions. For example:
Product Design Optimization
Design for Manufacturing (DFM)
Process development and validation
Regulatory compliance support (such as ISO 13485 or FDA registration)
Process standardization and quality system management
Whether it is a startup or a mature multinational company, CDMO can provide flexible support based on different needs.

The road to success in medical device manufacturing Series 4: Partners (Figure 2)
Why are more and more companies choosing CDMO?
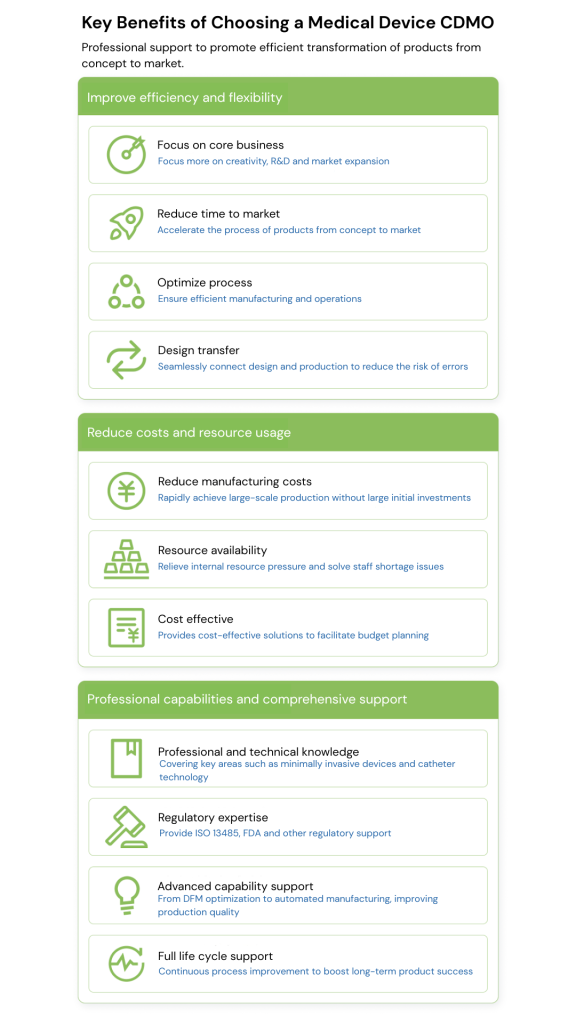
Key benefits of choosing a medical device CDMO include:
△ Focus
By handing over the design and manufacturing aspects to the CDMO, companies can focus on their core strategies: such as product creativity, R&D, marketing and fund raising.
△ Time to enter the market
CDMO has a mature team, standardized processes and rich experience, which can significantly shorten the conversion cycle from product concept to market and accelerate the time to market.
△ More efficient and lower-cost scale
The production scale can be expanded rapidly without large upfront investment; the experience and resources of CDMO can be used to reduce the risk of trial and error and achieve efficient and economical operation.
△ Professional knowledge
By working with a CDMO, companies gain direct access to a wide range of expertise, including:
Product areas: such as materials, minimally invasive medical devices, catheter technology, sensors, etc.
Process areas: validation testing, sterile packaging, manufacturing methods, etc.
△Advanced Capabilities
Your CDMO will also give you access to advanced capabilities from design innovation expertise to prototyping to manufacturing.
The road to success in medical device manufacturing Series 4: Partners (Figure 3)
Regulatory expertise
Staying abreast of regulatory knowledge is another important benefit of working with a CDMO. CDMOs begin their compliance efforts at the earliest stages of the product development process and continue them throughout the product lifecycle. This approach reduces the risk of regulatory challenges.
△ Certification and audit
The medical device CDMO will have an existing quality management system (QMS) with valid ISO 13485 certification and regulatory agency audit certification.
△DFM
DFM stands for Design for Manufacturing (or Design for Manufacturability). It involves designing a product in a way that not only provides the required performance characteristics but can also be manufactured commercially, i.e. efficiently and profitably with minimal quality risk.
△ Design transfer
Design handoff is the process of handing over a project from the design team to the operations team responsible for manufacturing the product. When you work with a CDMO, these teams will be within the same company, making the design transfer process more streamlined and reducing the risk of errors.
△Optimized process
A CDMO will have the experience to ensure the manufacturing process is fully optimized.
Resource availability
Many medical device companies struggle with resource availability, especially startups. This is where internal resources can become overstretched, leading to delays and other project challenges. Partnering with a CDMO will solve your resource availability issues.
△High cost-effectiveness
Working with a CDMO is a cost-effective way to design and manufacture medical device products, and it is also easier to budget and plan .
Flexibility
The best medical device CDMOs offer flexible working arrangements to ensure their services meet the different needs of different clients. It can reduce initial financial outlays and reduce project risks for clients.
△Knowledge Exchange
As medical device products become more advanced, there is a growing need to access specific, often highly niche expertise to solve problems and advance projects. Going out and finding expertise from the outset is difficult and time-consuming, whereas CDMOs already have established relationships with other specialized companies, facilitating faster knowledge sharing and keeping project timelines on track.
△The entire product life cycle
Partnering with a CDMO means you’ll benefit from dedicated support throughout your product lifecycle. The CDMO continually learns, adapts, and improves to make your product a success in the market.
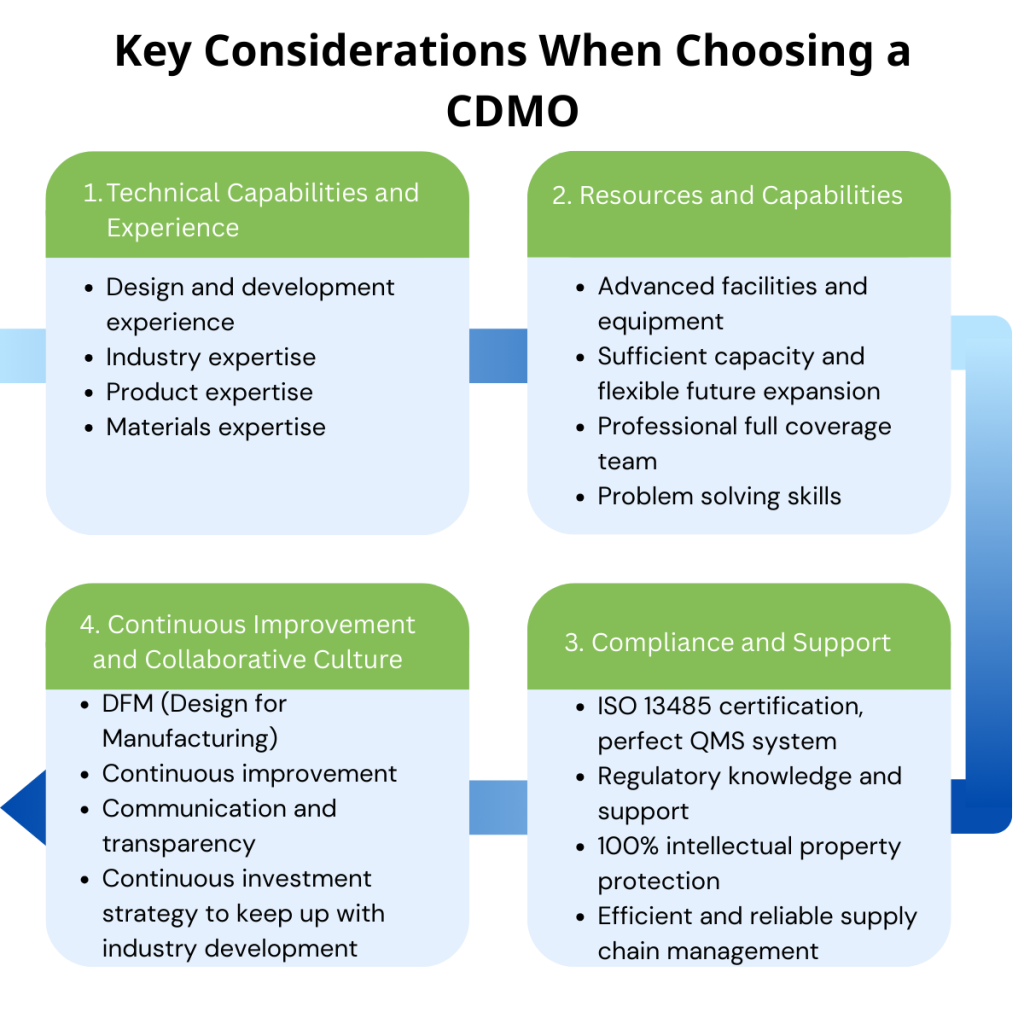
Key considerations for selecting a CDMO
So, how should we measure whether a CDMO is worth cooperating with? The following points cannot be ignored:
△Design and development experience
The key difference between a CDMO and a more traditional CMO (contract manufacturing organization) is that a CDMO will have design expertise and product development experience. It is also beneficial to select a CDMO with extensive product design and development experience. This includes experience managing complete projects, handling all aspects of design, and experience augmenting existing design teams with the development of specialized components.
△Industry expertise
The medical device industry is specialized and requires an unwavering focus on patient safety, quality, and compliance, so direct medical technology industry expertise is critical in a CDMO.
△Product expertise
It is very beneficial to work with a CDMO that has expertise in your product area. For example, if your product is a minimally invasive medical device, then ideally your CDMO should have expertise in minimally invasive medical devices.
△In line with your needs
Your CDMO should be closely aligned with your company’s specific needs, including:
Financial constraints: Ability to reasonably control budget and provide efficient services.
Milestone requirements: Ability to deliver each key node result on schedule.
Communication Preference: Provide transparent and clear communication mechanisms to ensure smooth flow of information.
A CDMO with strong adaptability will effectively reduce project risks.
△Capacity
Capacity is an often overlooked component of a medical device project, but it is essential. The CDMO you choose should have available capacity not only today, but also in the future. For example, you need to be confident that the CDMO has the capacity needed to design your product and then manufacture it when you are ready for large-scale production; the last thing you want is to be delayed due to capacity issues when you are about to commercialize your product.
The road to success in medical device manufacturing Series 4: Partners (Figure 4)
△ Facilities and equipment
A good CDMO has “”turnkey”” manufacturing capabilities, with advanced facilities and a wide range of equipment and resources, including clean room facilities, advanced manufacturing equipment, the latest technology platforms and skilled staff, as well as multi-link support such as assembly, packaging and sterilization capabilities.
△ Investment strategy
The CDMO’s investment strategy should also be factored into your decision making. For example, whether your CDMO regularly invests in its capabilities will determine its competitiveness:
Staff training
Technology Upgrade
Device Updates
CDMOs that fail to keep pace with industry developments will find it difficult to meet customer needs in the long term.
△Problem-solving ability
Medical device products are becoming increasingly complex through innovative designs, miniaturization, complex components, and/or inclusion of advanced technologies such as sensors. Your medical device CDMO should have extensive problem-solving capabilities and a proven track record to ensure they can meet your requirements.
△Material expertise
The materials used to produce new medical device products are critical to patient safety, usability, manufacturability, quality consistency, raw material reliability, and, increasingly, sustainability. A CDMO with material science expertise can provide an additional level of assurance for product quality.
△DFM (Design for Manufacturing)
DFM is an important process throughout the entire design phase to ensure that the product can enter large-scale commercial production efficiently and stably. The goals of DFM include reducing the number of components and minimizing the complexity of the assembly process. A CDMO that masters the DFM methodology will significantly increase the success rate of the project.
△ Certification
Your medical device CDMO should have an established quality management system (QMS) and be certified to ISO 13485. It should also be audited by regulatory agencies and have valid certifications.
Regulatory knowledge and support
Regulatory compliance is a requirement throughout the medical device lifecycle. Therefore, regulatory knowledge and support are important considerations when selecting a CDMO. Your CDMO should have quality and regulatory resources to provide you with the support you need, as well as a complete and robust document control process.
Supply Chain
Given the disruptions many supply chains have faced over the past few years, supply chain management is more important than ever. The CDMO you choose should have advanced and efficient supply chain management processes, including strong oversight, to ensure your supply chain runs smoothly.
△Continuous improvement
Continuous improvement is also an important criterion for measuring whether a CDMO has long-term cooperation value. Not only should they establish production processes to mass produce medical devices, they should also have:
Regularly optimize manufacturing processes;
Support enterprises’ continuous innovation and improvement in subsequent product iterations, such as improving efficiency or reducing costs.
△Communication and transparency
Communication is important in any business relationship, especially one as close and commercially sensitive as the one between a medical device company and a CDMO. Communication should be honest and open, with full transparency.
△IP protection
It is also important that your IP is always protected and 100% ownership of your IP should be maintained at all times. Confidentiality is a non-negotiable component.
△Team
Finally, it is equally important to understand the CDMO team: leadership, design, quality, and production. Do they have a good interaction and partnership with you? These may sound like low-level considerations, but they are critical.
Choose the right CDMO to create the future of medical devices
The role of CDMOs has long surpassed simple production support. They are becoming the core force for medical device companies to achieve innovation, optimize costs and improve efficiency. When choosing a CDMO, you should not only pay attention to its technology, facilities and team strength, but also examine its strategic vision, long-term cooperation potential and responsiveness to customer needs. Finding the right partner will also enable your medical device products to enter the market faster and more safely, and gain the opportunity for continued success.
Reference: Whitepaper-Three Essential Phases of Medical Device Manufacturing Success