The race to develop safer, smarter, and more personalized medical devices is faster than ever. Manufacturers face growing pressure to deliver high-performance, connected, and affordable products that improve patient outcomes while meeting strict regulatory demands.
At the same time, clinicians expect devices that simplify procedures, shorten treatment times, and integrate seamlessly into their workflows. The result? A rapidly evolving industry where innovation speed and reliability have become key competitive advantages.

The Complexity Challenge
Modern medical devices combine advanced electronics, software, sensors, and connectivity into compact systems that must perform flawlessly. This complexity introduces both technical risk and design uncertainty, from system integration challenges to regulatory compliance hurdles.
To manage this, developers need tools that enable faster, smarter iteration, long before the first prototype is built.
Enter Computational Modeling and Simulation (CM&S)
Computational Modeling and Simulation (CM&S) has emerged as a powerful toolset for accelerating device development. By replicating the real-world behaviour of a design in a virtual environment, teams can explore design alternatives, identify weaknesses, and optimize performance before physical testing even begins.
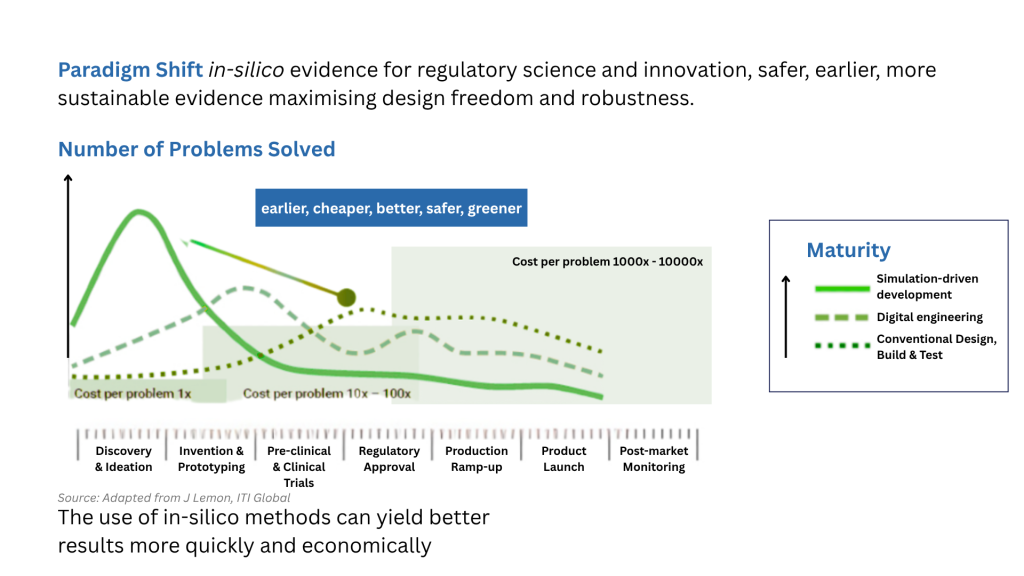
Key advantages include:
- Reduced cost and time-to-market: Virtual iterations replace multiple physical builds, shortening the design–procurement–test cycle.
- Improved reliability: Simulations help predict failures and optimize performance under various conditions.
- Regulatory confidence: Data-driven insights support more efficient validation and documentation for submissions.
Real-World Applications of CM&S in Medical Device Design
Here are five examples of how CM&S is transforming device development today:
1. Worst-case analysis in articulated components
Articulated components, such as surgical instruments and imaging positioning tools, must operate reliably under a variety of motion, load, and imaging conditions. Identifying the “worst-case” scenario (i.e., the performance of the device under test under maximum stress, strain, or displacement) is crucial for ensuring long-term performance and safety. Computational modeling can provide detailed analysis of these scenarios without requiring extensive physical testing.
Case Study: Simulations
Using programming scripts and structural finite element analysis (FEA) to explore thousands of device orientations and configurations for surgical positioning guides . The complex geometry of the guides and the highly variable positioning of components make worst-case scenarios difficult to determine. Traditional methods (principles-based or experimental analysis) are more labour-intensive and time-consuming, slowing down projects and increasing costs.
Result:
The simulation efficiently identified the worst-case scenarios for critical components and validated the model results through limited experiments, thereby accelerating the testing and regulatory submission process and increasing team confidence.
2. Key interfaces for sealing test
Many medical devices require sealed interfaces to ensure leak-proof protection, such as drug delivery systems, diagnostic equipment, and implantable devices. Failure of the seal can lead to significant problems and equipment malfunctions, impacting the safety and effectiveness of the device.
Case Study:
Structural finite element analysis was used to simulate the stress and deformation of the O-ring and surrounding interface material under various extrusion conditions . Some noteworthy aspects include:
- Excessive compression of the O-ring may cause plastic deformation, thereby affecting the leakage pressure threshold.
- The interface material used to secure and compress O-rings is not entirely rigid and bends due to the pressure from the O-rings. This can be addressed by reducing the contact on the O-rings or excessive stress in these materials.
- There is a non-uniform force distribution along the O-ring interface, which is difficult to approximate using basic principles. Therefore, using a model for evaluation is the best choice.
Results:
By modelling with accurate material properties and representative O-ring geometries (e.g., contoured O-ring grooves), sealing performance and identified potential failure points were predicted . Insights from these simulations led to design modifications, selective interfacial material thickening and optimization of the extrusion percentage on the O-ring, improving the reliability and durability of the seal and interface. This further reduced the likelihood of needing to iterate on physical builds and testing, and mitigated risk before in-situ testing and equipment use.
3. Thermal Management
Effective thermal management is crucial for many medical devices that contain electronic components (high-frequency driven devices, diagnostic devices, and some implantable devices). Overheating can affect the device’s functionality and safety.
Case Study:
In a laboratory diagnostic device used for respiratory analysis, computational fluid dynamics (CFD) and thermal simulation (solid-state and fluid) are employed to analyze the system’s thermal effects . The main principle approximates the power level required to maintain the system at a specific temperature to keep the gas sensor calibrated and prevent condensation inside the patient’s breath sample.
Result:
CFD provides identification capabilities, enabling more accurate simulation of heat generation, steady-state heat dissipation, and other factors within equipment. Simulations pinpoint areas where heat build-up could damage components or impact equipment performance, guiding heatsink selection and material validation. This improves equipment reliability and reduces the risk of failure.
4. Structural optimization to reduce failures
Optimizing equipment structural design is an important step in ensuring that it can withstand various stresses during operation and reducing the risk of failure.
Case Study:
In a typical case study, the primary focus was on improving the geometry of a neurosurgical cannula. Because the cannula is attached to the top of the device, the loop cannula carries a higher risk of catching the cannula when a clinician brushes past the device. The inability to use robust metal tubing in MRI imaging environments necessitates the use of brittle, non-magnetic ceramics as structural components, further increasing the chance of breakage. These issues can all occur during device implantation and use.
Structural finite element analysis (FEA) was used to simulate various load conditions and orientations to determine the stress distribution that could lead to fracture or other failures. Through iterative design, the maximum stress in the geometry was compared and correlated with a safety factor and the ultimate tensile strength of the brittle ceramic to find the optimal design.
Results:
The optimized design significantly improved strength and durability. It reduced the likelihood of device insertion/use failure and the need for subsequent corrective procedures, thereby improving patient safety and device reliability.
5. Drop test for medical equipment
For medical devices, drop testing is a crucial step in assessing their ability to withstand impacts or accidental drops throughout their lifespan . The mobility classification under IEC 60601-1 defines the types and conditions of the tests. However, direct physical testing can lead to damage to expensive components and lengthy iteration cycles.
Case Study:
CM&S enables drop and impact testing in less time and with less effort, while predicting future impacts and allowing for agile design changes before ordering and building. It also allows for a deeper understanding of the impact of drops on components. Beyond the physically observed failures of traditional testing, the impact of failure risks on materials and components can be reviewed, giving teams greater confidence before formal equipment testing, thereby reducing project risk, timelines, and costs.
Result:
Simulations revealed the stress distribution caused by the impact and highlighted weak points in the fastened areas, including between housings and inside components. This prompted design adjustments to improve shock resistance and component/housing retention forces, ensuring reliable performance even after accidental drops. It also provides users with greater confidence in the safety of their equipment.
Accelerating Innovation Through Virtual Design
These use cases show how CM&S is transforming medical device development from reactive testing to proactive innovation. With detailed, validated virtual models, manufacturers can make confident design decisions earlier—shortening timelines, cutting costs, and improving patient safety.
As computing power continues to grow, the influence of CM&S will only deepen, driving the next generation of smarter, safer, and more efficient medical technologies.
Source: https://www.mpo-mag.com/exclusives/5-ways-simulation-in-medical-device-design-accelerates-innovation/
